Quarterly insights: Internet of Things
Shift to condition monitoring to finally drive connected pallet adoption
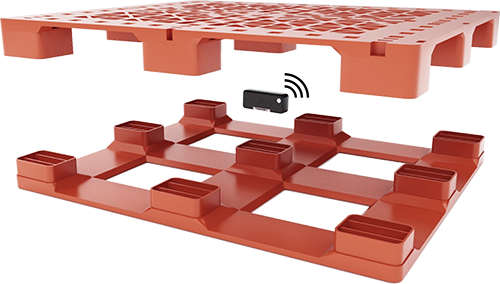
The first generation of IoT solutions for wirelessly connecting pallets focused mainly on the value of the pallets themselves, only tracking location. This approach failed to gain traction because it offered insufficient return on investment, addressed too small a portion of the market, and suffered from high up-front costs and ongoing technology maintenance costs.
A second generation of IoT pallet technology is emerging that overcomes these shortcomings. These solutions monitor the conditions pallets – and more importantly, their cargoes – experience on their journeys through supply chains. By monitoring conditions, such as temperature, vibration, shock and humidity, these solutions provide information that helps manufacturers, distributors, and shippers preserve the products pallets carry, which are typically much more valuable than the pallets themselves.
We expect these solutions’ broad and substantial appeal to help drive strong growth for companies providing second-generation pallet IoT solutions, and we profile several such companies.
TABLE OF CONTENTS
Includes discussion of eight private companies
- Pallets: A pillar of modern logistics
- Massive opportunity explains continued interest in digitizing pallets
- Connected pallet 1.0: The first generation of connected pallet technology
- Why connected pallet 1.0 failed
- Connected pallet 2.0: More than just location tracking
- Why connected pallet 2.0 will succeed
- Characteristics useful in predicting success
- Representative companies
- Serving a more palatable IoT pallet solution
- IoT index remains range-bound
- IoT M&A: Notable transactions include Vivint, Noonlight, Compology, Sierra
- IoT private placements: Notable transactions include Memfault, Blues Wireless, Netradyne, and CoolR
Pallets: A pillar of modern logistics
Clinical trial supplies are the investigational medicinal products and other materials and equipment needed to effectively conduct clinical trials. This includes everything from drugs being tested to patient biological samples to the packaging and labeling used in moving them from one location to another. Managing clinical trial supplies involves a range of activities, from planning and forecasting to procurement and distribution. Clinical trial supply chains are deeply interconnected. Expected enrollment levels at trial sites affect supply and logistics decisions made upstream. Unfortunately, most teams throughout the process work in silos, making clinical trial supply management extremely complex and difficult with challenges that include managing inventory levels, ensuring timely delivery of investigational drugs, and maintaining regulatory compliance and patient safety.

Request full report
To access the full report, please provide your contact information in the form below. A First Analysis representative will follow up with you shortly. Thanks for your interest in First Analysis research.
